HIV Associated Facial Lipoatrophy
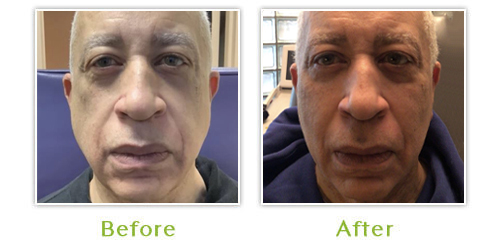
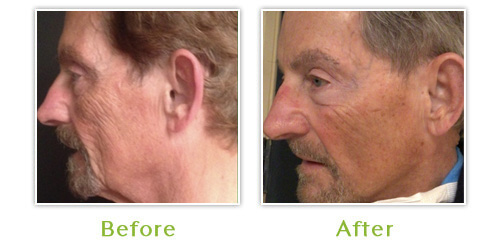
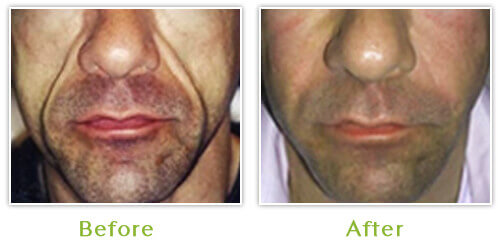
New advances in antiviral therapy have enabled HIV/AIDS patients to live long and productive lives; however, this therapy is not without adverse events. Fat loss and redistribution of fat, commonly seen with facial lipoatrophy, has rapidly become the hallmark for Highly Active Anti-Retroviral Therapy (HAART). Recent studies have shown that most patients treated with HAART will develop lipodystrophy within one year from the initiation of treatment.
The psychological and social impact of facial lipoatrophy is quite considerable leading to anxiety and depression in many individuals. As a result, some patients have become non-compliant with anti-retroviral therapy to avoid the inevitable facial wasting. Most unsettling of all, individuals have reported that they have been forced into disclosing their HIV status due to their anorexic facial appearance.
Dr. Burgess, Medical Director of the Center for Dermatology and Dermatologic Surgery and Board-certified dermatologist, has been treating HIV associated lipoatrophy since 2001 and has treated countless patients from all over the United States. She finds that patients with severe facial wasting require 3 sessions on the average to obtain 90 to 100 percent dermal enhancement. With the use of topical anesthetic for very little discomfort, patients tolerated the procedure well without significant side effects or down time. The most common side effect is bruising, which may persist for seven to ten days.
Poly-L-lactic Acid (Sculptra™) has emerged as one of the most talked about fillers for the treatment of facial lipoatrophy. It was first approved and popularized in Europe in 1999 for medical treatment of facial lipoatrophy in HIV infected individuals. The United States Food and Drug Administration review panel unanimously agreed for FDA approval in August 2004.
Cosmetic enhancement/Liquid facelift

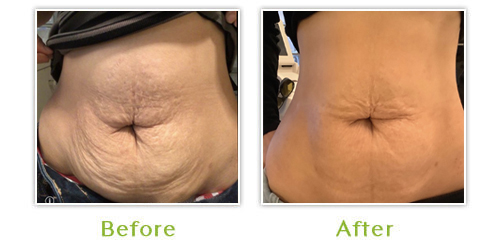
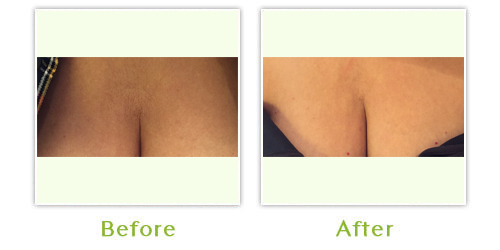
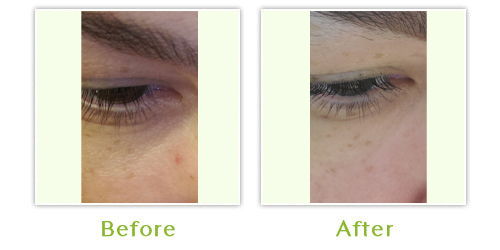
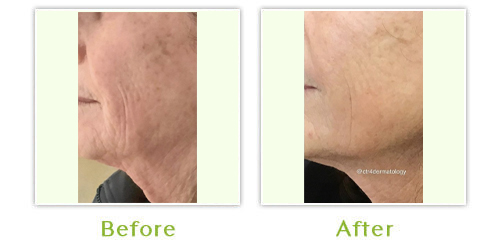
Sculptra is a bio-stimulatory product, which causes the skin to produce and remodel collagen. It is an injectable treatment, which restores lost facial volume to reduce the appearance of wrinkles, sagging skin and sunken cheeks. Results develop gradually, after about four weeks and may last up to two years. Sculptra is safe and biocompatible, so pre-treatment allergy testing is not needed. It may also be administered with topical anesthetic.
In Europe, treatment indications include scars, furrows, creases, fine lines and wrinkles and to increase tissue volume necessary to treat conditions such as facial lipodystrophy (loss of fat).
As a Board-certified dermatologist, Dr. Cheryl Burgess has performed studies in the best techniques for using Juvéderm® and Restylane®. She is also a Clinical Investigator for Allergan and Merz. Call (202) 955-5757 and schedule an appointment at Center for Dermatology and Dermatologic Surgery today.